GLUCOJECT
Glucoject Insulin Pen Needle, Length 4 mm, Gauge 32, 100 Pieces
Glucoject Insulin Pen Needle, Length 4 mm, Gauge 32, 100 Pieces
Secure payment
Discreet and sanitized package
Fast and safe shipping
Description
Description
Glucoject
Pen Needles
Insulin pen needles
PRODUCT IDENTIFICATION DATA
| Trade name | Glucoject Pen Needles |
| Manufacturer | HTL-STREFA SA, 95-035 Ozorkòw, 7 Adamòwek St., Poland. |
| Distributor | A.Menarini Diagnostics Florence - Italy |
| Internal Codes | 44029-44030-44031-44032-44033-46789 |
| CND Code | A010199 - Insulin Pen Needles |
| Year of placing on the market | 2014 |
PRODUCT COMPLIANCE WITH APPLICABLE DIRECTIVES
| European Directive 93/42/EEC | Compliant - CE Medical Devices (annex V + VII) |
| Classification | Class IIa |
| Notified body | DEKRA |
GENERAL CHARACTERISTICS
INFORMATION ON THE REFERENCE CLASS
| General product description | Glucoject® Pen Needles are sterile, single-use needles intended for use with insulin pens to administer medications into the body. Specifically, they include: - High quality needles - Thin-wall technology - New lubrication method - Screw application - 100% sterile - Six different needle versions |
| Components | - Needle - Internal protective hood - External protective hood - Seal |
| Characteristics | - Extremely sharp needle tip - Triple precision bevel - Special electropolishing - Super thin wall - Exclusive silicone lubrication |
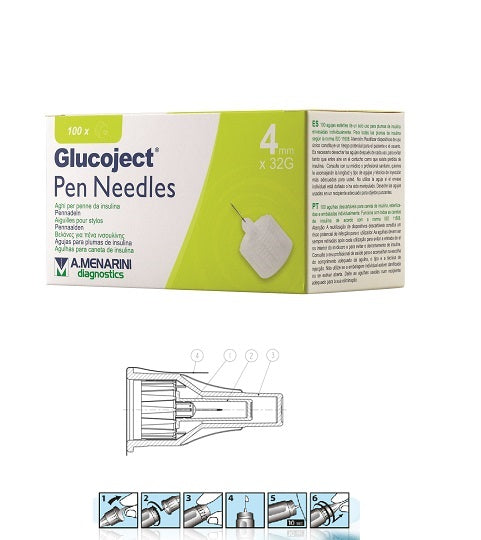
| Technical drawing (figure 2) | 1) Needle complex 2) Needle cover 3) Protective hood 4) Seal |
| Dimensions | Length: 29.1 mm Width: 16.5 mm Depth: 16.8 mm |
| Use | Glucoject® Pen Needles are sterile, single-use insulin pen needles intended for use in conjunction with injection pens to deliver medications (usually insulin) into the body. |
| Reuse | Glucoject® Pen Needles are sterile and disposable. Sterility is guaranteed if the needle seal is intact. Reuse can: - cause the needle to break; - cause lipohypertrophy; - damage the sharpness or may cause the tip to bend, resulting in bleeding, bruising or scarring; - increase the risk of needle breakage in the skin; - increase the risk of infection as the needle is no longer sterile. |
| Method of use and mechanism of action (figure 3) | 1. Remove the seal from the needle. 2. Place the needle straight onto the pen and screw it on until it's secure. Remove the outer protective cap and set it aside. You'll need it later to remove the needle from the pen. Pull off the inner protective cap. 3. Set the insulin dose. 4. Check the flow by pricking the needle in the air to release a small amount of fluid. A small drop should appear at the needle tip. 5. Perform the injection. 6. Replace the outer protective cap and remove the needle from the pen. |
| Insulin administration | - It is essential to choose an appropriate site on the body where to inject insulin. - Insulin penetrates at different rates depending on the injection site. - It is recommended to inject insulin in the same area for the best results, but rotating injection sites is important to avoid side effects. Your doctor or healthcare professional should recommend the most appropriate injection site. For example: - For rapid-acting analogues and short-acting regular insulins, the best place is the abdomen. - For long-acting analogues and regular NPH insulins, the best place is the thighs. - For premixed analogues and mixed human insulins, the best places are the abdomen and upper arm. |
| Validity period | Shelf life is associated with the duration of sterility. Based on tests conducted by the manufacturer HTL-STREFA SA, whose data is archived in accordance with the Quality Management System procedures required by the PN-EN ISO 13485 standard, a warranty of 3 years from the date of manufacture is guaranteed. |
| Recommendations | - Glucoject® Pen Needles allow the administration of insulin to adults, children and obese patients. - Obese people should use longer needles than leaner people. If a lean person uses a long needle, there's a risk of injuring muscle tissue. - Generally, if a heavy person uses a short needle, the insulin may tend to leak around the injection site, thus causing an incorrect dosage. Pinching the skin before injecting helps avoid these problems, and anyone can do it. - As for pain, a thick needle can cause more pain than a thin one, but the perception of pain varies greatly from person to person. - In case of high doses of insulin it is better to use a thicker needle. - It is also important to use an appropriate injection technique. - Insulin delivery techniques vary depending on the injection site and the length of the pen needle. |
| Information about the instrument components | Material Components Needle/cannula AISI 302 or 304 stainless steel Polypropylene container Polypropylene needle cover Safety cap Polypropylene Patterned Lacquer Seal Corrugated cardboard packaging box |
| Sterilization | Gamma irradiation performed in accordance with PN-EN ISO 11137 |
| Learn more | - Does not contain natural latex - Does not contain PVC - Does not contain phthalates |
COMMERCIAL PACKAGING
| Product code | Repertoire Number | Commercial Name | Specifications Depth x Caliber (mm x G) | Packaging |
| 44032 | 1195790 | Glucoject Pen Needles | 10 mm x 29 G | 100 pcs. |
| 44033 | 1195791 | Glucoject Pen Needles | 12 mm x 29 G | 100 pcs. |
| 46789 | 1195782 | Glucoject Pen Needles | 5 mm x 31 G | 100 pcs. |
| 44030 | 1195787 | Glucoject Pen Needles | 6 mm x 31 G | 100 pcs. |
| 44031 | 1195789 | Glucoject Pen Needles | 8 mm x 31 G | 100 pcs. |
| 44029 | 1195734 | Glucoject Pen Needles | 4mm x 32G | 100 pcs. |
All Glucoject Pen Needles are suitable for use with all insulin pens according to ISO 11608
GLUCOJECT
Glucoject Insulin Pen Needle, Length 4 mm, Gauge 32, 100 Pieces
Glucoject Insulin Pen Needle, Length 4 mm, Gauge 32, 100 Pieces
Couldn't load pickup availability
Is this your first order?
We offer a hassle-free return policy, so you can shop with confidence. Fast and reliable shipping ensures your products arrive directly to your door. And don't worry about privacy : your data is safe with us. We're here to make your online shopping a stress-free and convenient experience.
Sign up for our newsletter. We only send useful stuff. We promise 😉
Product images are purely indicative and may not be fully representative of the packaging and product features, as they may vary in color, size, or content. Any decorations, gift wrapping, and items included in the images for product presentation purposes will not be shipped with orders. Product names, ingredients, and percentages indicated in the descriptions are purely indicative and may be subject to change or updates by the manufacturers. Due to the impossibility of updating these updates in real time, the photos and technical information of the products listed on farmaInnovation.it may differ from those shown on the label or otherwise disclosed by the manufacturers. The only identifying element is the MINSAN (Ministry of Health) code. Farmacia Barni, farmainnovation.it , does not guarantee the accuracy and timeliness of the information published and declines all responsibility for any errors, omissions, or failure to update it. farmainnovation.it assumes no responsibility for damages of any kind that may arise from accessing the published information.

