ONILAQ
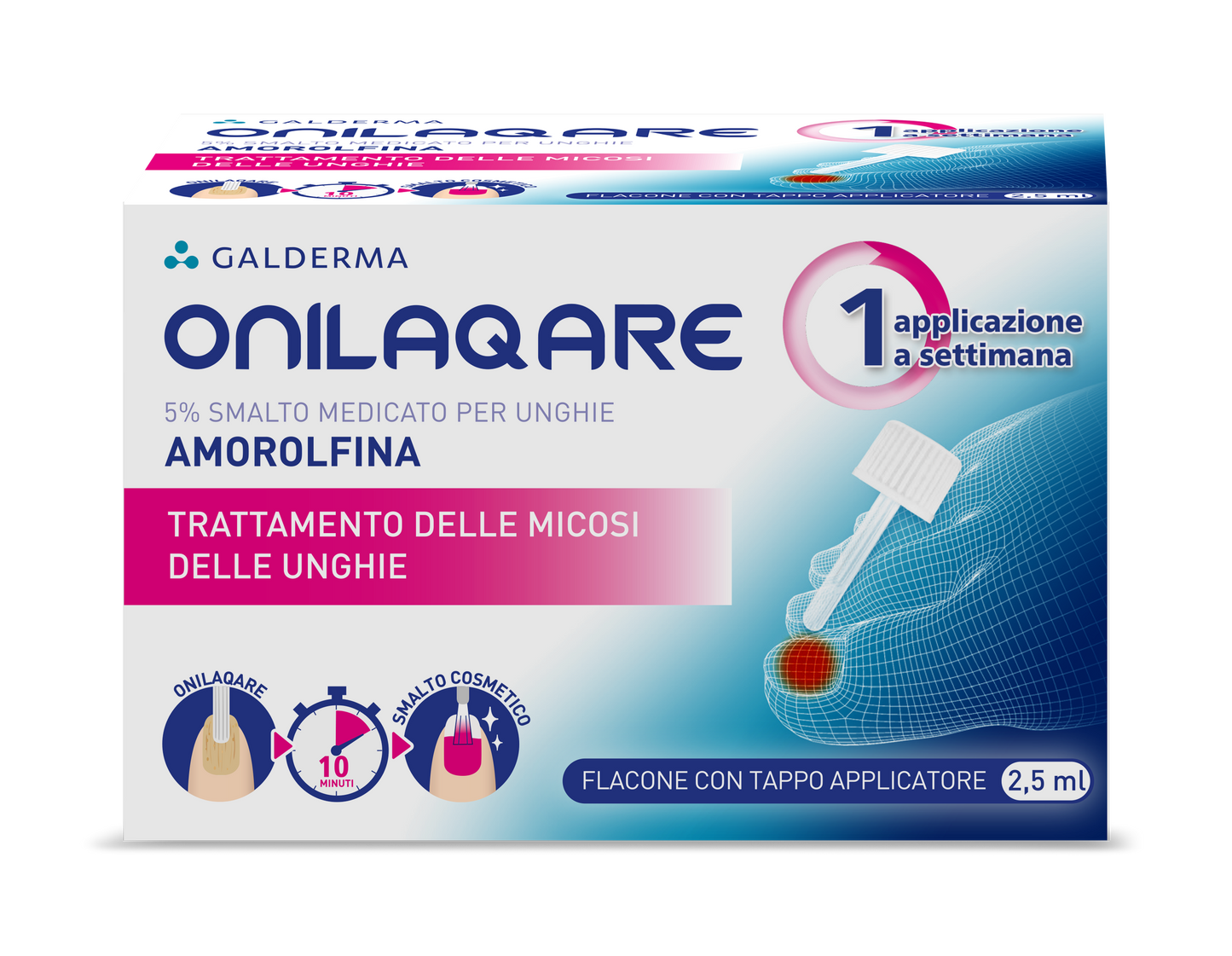
ONILAQARE 5% MEDICATED NAIL POLISH 1 BOTTLE OF 2.5 ML WITH APPLICATOR CAP
ONILAQARE 5% MEDICATED NAIL POLISH 1 BOTTLE OF 2.5 ML WITH APPLICATOR CAP
Secure payment
Discreet and sanitized package
Fast and safe shipping
Description
Description
Onilaqare 5% medicated nail polish
Amorolfine
What is it and what is it used for?
ONILAQARE 5% medicated nail polish contains amorolfine, an active substance capable of inhibiting the growth of fungi (antifungal).
It is used for the local treatment of onychomycosis (nail infections caused by fungi) in adults involving no more than two nails and affecting the lateral or upper part of the nail, as shown in photo 1. If the infection looks more like photo no. 2 or 3, you should consult your doctor before using Onilaqare 5% medicated nail polish.
A nail fungus usually causes discoloration of the nail (white, yellow or brown) and thickening, although the appearance can vary considerably, as shown in the photographs.
What you need to know before taking the medicine
Do not use Onilaqare 5% medicated nail polish
- if you are allergic (hypersensitive) to the active substance (amorolfine) or to any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor or pharmacist before using Onilaqare 5% medicated nail polish:
• if you suffer from diabetes;
• if you are being treated for immune system disorders (a disorder that reduces the body's defenses);
• if you suffer from circulatory problems in your hands and feet;
• if you have a history of nail injury, skin conditions such as psoriasis, or other chronic skin condition, swelling, yellow nails associated with breathing problems, painful nails, distorted/deformed nails or any other disorder in the nail area;
• if the nail is severely damaged (with lesions covering more than two-thirds of the nail plate) or infected. In these cases, your doctor may prescribe oral therapy in combination with medicated nail polish.
Do not apply Onilaqare 5% medicated nail polish to the skin around the nail.
Avoid contact with mucous membranes (e.g., mouth or nostrils). Do not inhale the nail polish.
If Onilaqare 5% medicated nail polish comes into contact with your eyes or ears, rinse immediately with water and contact your doctor, pharmacist, or nearest hospital straight away.
In case of contact with organic solvents (for example nitro thinners, turpentine, etc.) wear waterproof gloves to protect the layer of Onilaqare 5% medicated nail polish applied to the nails.
Do not use the nail file used for diseased nails on healthy nails.
Do not use artificial nails during treatment with Onilaqare 5% medicated nail polish.
After applying Onilaqare 5% medicated nail polish, an interval of at least 10 minutes must be respected before using any cosmetic nail polish.
Before repeating the application with Onilaqare 5% medicated nail polish, the cosmetic nail polish must be carefully removed.
This medicine may cause allergic reactions, some of which can be serious. If this occurs, stop using the medicine, remove it immediately with nail polish remover or the cleansing pads provided, and consult a doctor. The medicine should not be reapplied.
If you experience any of the following symptoms, contact your doctor urgently:
- has difficulty breathing
- his face, lips, tongue or throat swell;
- his skin shows a severe rash.
Children and adolescents
The use of Onilaqare 5% medicated nail polish is not recommended in children and adolescents up to 18 years of age.
Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Reactions to this medicine are rare.
Some nail disorders (e.g., nail discoloration, nail breakage, or nail brittleness) have been reported. However, these reactions may be directly related to nail infection.
The following side effects are listed in order of frequency:
Rare: May affect up to 1 in 1,000 people
• Nail disorders, discoloration and brittleness of the nails
Very rare: may affect up to 1 in 10,000 people
• Burning sensation of the skin
Frequency not known: frequency cannot be estimated from the available data
• Systemic allergic reaction (a serious allergic reaction that may be associated with swelling of the face, lips, tongue or throat, difficulty breathing and/or a severe skin rash)
• Redness (erythema)
• Itching
• Inflammatory skin reaction (contact dermatitis)
• Hives
• Blisters
Reporting of side effects
If you experience any side effects, including any not listed in this leaflet, talk to your doctor or pharmacist. You can also report side effects directly via the national reporting system at: https://www.aifa.gov.it/content/segnalazionireazioni-avverse .
By reporting side effects you can help provide more information on the safety of this medicine.
ONILAQ
ONILAQARE 5% MEDICATED NAIL POLISH 1 BOTTLE OF 2.5 ML WITH APPLICATOR CAP
ONILAQARE 5% MEDICATED NAIL POLISH 1 BOTTLE OF 2.5 ML WITH APPLICATOR CAP
Couldn't load pickup availability
Is this your first order?
We offer a hassle-free return policy, so you can shop with confidence. Fast and reliable shipping ensures your products arrive directly to your door. And don't worry about privacy : your data is safe with us. We're here to make your online shopping a stress-free and convenient experience.
Sign up for our newsletter. We only send useful stuff. We promise 😉
Product images are purely indicative and may not be fully representative of the packaging and product features, as they may vary in color, size, or content. Any decorations, gift wrapping, and items included in the images for product presentation purposes will not be shipped with orders. Product names, ingredients, and percentages indicated in the descriptions are purely indicative and may be subject to change or updates by the manufacturers. Due to the impossibility of updating these updates in real time, the photos and technical information of the products listed on farmaInnovation.it may differ from those shown on the label or otherwise disclosed by the manufacturers. The only identifying element is the MINSAN (Ministry of Health) code. Farmacia Barni, farmainnovation.it , does not guarantee the accuracy and timeliness of the information published and declines all responsibility for any errors, omissions, or failure to update it. farmainnovation.it assumes no responsibility for damages of any kind that may arise from accessing the published information.

