GIMA
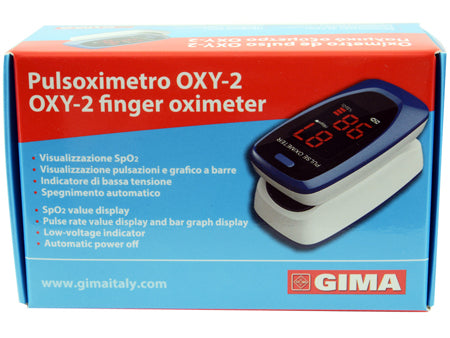
OXY-2 FINGER PULSE OXIMETER LED SCREEN 60X30.5X32.5MM 1 PIECE
OXY-2 FINGER PULSE OXIMETER LED SCREEN 60X30.5X32.5MM 1 PIECE
Secure payment
Discreet and sanitized package
Fast and safe shipping
Description
Description
OXY-2 PULSE OXIMETER
The Finger Pulse Oximeter is a non-invasive device intended for the instantaneous monitoring of arterial hemoglobin saturation (SpO2) and heart rate of adults and children in home and hospital environments (including clinical use in internal/surgical therapy, anesthesia and intensive care, etc.).
It is not intended for continuous monitoring.
It's compact, easy to use and carry, and has low power consumption. The two supplied AAA batteries provide continuous use for 24 hours.
Auto power off when no signal is received within 5 seconds.
Low battery indicator as a flashing battery icon.
The patient simply inserts his fingertip into the device's photoelectric sensor and the Hemoglobin Saturation value immediately appears on the screen.
Technical specifications :
- Display format: Digital tube display;
- Screen type: LED;
- SpO2 measuring range: 0%-100%;
- Heart rate measurement range: 30 bpm - 250 bpm;
- Heart rate intensity display: bar display;
- Power requirements: 2 x 1.5V AAA alkaline batteries; adjustable range: 2.6V - 3.6V;
- Supply current: less than 25 mA;
- Resolution: 1% for SpO2 and 1 bpm for heart rate;
- Measurement accuracy: ±2% in the 70%-100% SpO2 phase, and irrelevant when the phase is lower than 70%. ±2 bpm or ±2% (select whichever is larger) for heart rate;
- Measurement performance under low filling pressure: SpO2 and heart rate are displayed correctly when the heart rate-filling ratio is 0.4%. SpO2 error ±4%, heart rate error ±2 bpm or ±2% (select whichever is larger);
- External light tolerance: The difference between the value detected in the presence of artificial light, internal natural light and that of a dark room is less than ±1%;
- It is equipped with a function switch. The pulse oximeter turns off if it does not detect any fingers in the device;
- Optical sensor: red light (wavelength is 660 nm, 6.65 mW) and infrared (wavelength is 880 nm, 6.75 mW);
- Operating environment:
Temperature: 10°C - 40°C
Relative humidity: ≤75%
Atmospheric pressure: 700 hPa - 1,060 hPa.
Note: The optical sensor is a light-emitting component that influences other medical devices by applying the same wavelength range.
How to use
Insert the two batteries in the correct orientation and then replace the cover. Open the sensor. Have the patient insert the finger into the rubber-coated sensor (make sure the finger is in the correct position), then let the sensor close on the finger. Press the power button on the front panel. Do not move the finger and keep the patient at rest during the measurement. Furthermore, the patient is advised not to move. The measurement results are displayed directly on the display.
In the recorder startup state, press the button and the device will be reset.
The nails and the glow tube must remain on the same side.
Cleaning and maintenance : Replace the batteries when the low battery indicator starts flashing.
Clean the surface of the device before use. Wipe with medical-grade alcohol first and then let it air dry or wipe dry.
Use medical grade alcohol to disinfect the product after use, preventing contamination for next use.
If you will not use the Pulse Oximeter for a long time, remove the batteries.
The device must be calibrated on a regular basis (or in accordance with the hospital's calibration schedule).
Do not sterilize the device with high pressure.
Warnings
The finger must be positioned appropriately, otherwise this may cause inaccurate measurements.
The SpO2 sensor and photoelectric receiving tube should be arranged so that the patient's arteriole is in a position between the two.
The SpO2 sensor should not be used at a site or on a limb strapped with a blood pressure or cuff or during an intravenous injection.
Make sure the optical path is free of optical obstructions, such as rubberized fabric.
Excessive ambient light can affect the measurement result. This includes fluorescent lamps, double red lights, infrared radiators, direct sunlight, etc.
Even strenuous patient action or extreme electrosurgical interference can influence accuracy.
The patient undergoing the examination cannot use nail polish or other cosmetics.
Do not immerse the appliance in any type of liquid.
It is recommended to keep the device in a dry environment. Humidity could reduce the lifespan of the device or even damage it.
Explosion Hazard: Do not use the Pulse Oximeter in environments containing flammable gases such as some flammable anesthetic agents.
DO NOT use the Pulse Oximeter while the patient is undergoing an MRI or CT scan.
Please check the package before use to ensure that the device and its accessories fully comply with the packing list, otherwise the device may malfunction.
Please do not measure this device with paper function tests for the relevant device information.
Keep the pulse oximeter away from dust, vibration, corrosive substances, explosive materials, high temperatures and humidity.
If the pulse oximeter gets wet, please stop using it.
When transported from a cold environment to a warm or humid environment, do not use it immediately.
DO NOT press the front panel buttons with sharp materials.
Disinfection of the pulse oximeter with high-temperature steam or pressure is not permitted. Do not immerse the pulse oximeter in liquids. When cleaning, wipe the surface with a soft cloth soaked in medical-grade alcohol. Do not spray liquids directly onto the device. When cleaning the device with water, the temperature must be below 60°C.
Since fingers that are too thin or too cold may affect the patient's normal SpO2 and heart rate measurement, place thicker fingers, such as the thumb and middle finger, deep enough into the device.
Do not use the device on children or infants. The device is suitable for children over four years old and adults (weighing between 15 and 110 kg).
The device may not work for all patients. If you are unable to achieve stable measurements, discontinue use.
The data update period is less than 5 seconds: this duration varies according to different individual heart rates.
If any abnormality appears on the screen during the test procedure, remove your finger and reinsert it to restore normal use.
The normal life cycle of the device is three years from the first time it is turned on.
The support lanyard attached to the product is made of hypoallergenic material. If a particular group is allergic to the support lanyard, discontinue use. Also, be careful when using the support lanyard; do not hang it around the patient's neck to avoid injury.
The device does not have a low voltage alarm function; it only indicates the battery level. Please replace the battery when the battery is depleted.
The device does not have an alarm function for particularly low parameters. Do not use the device in situations where alarms are required.
Batteries must be removed if the device is to be stored for more than a month, otherwise they may leak.
A flexible circuit connects the two parts of the device. Do not twist or pull the connection.
Conservation
Store between -40°C and 60°C. Relative humidity: ≤95%
Format
1 piece.
Code 35072
GIMA
OXY-2 FINGER PULSE OXIMETER LED SCREEN 60X30.5X32.5MM 1 PIECE
OXY-2 FINGER PULSE OXIMETER LED SCREEN 60X30.5X32.5MM 1 PIECE
Couldn't load pickup availability
Is this your first order?
We offer a hassle-free return policy, so you can shop with confidence. Fast and reliable shipping ensures your products arrive directly to your door. And don't worry about privacy : your data is safe with us. We're here to make your online shopping a stress-free and convenient experience.
Sign up for our newsletter. We only send useful stuff. We promise 😉
Product images are purely indicative and may not be fully representative of the packaging and product features, as they may vary in color, size, or content. Any decorations, gift wrapping, and items included in the images for product presentation purposes will not be shipped with orders. Product names, ingredients, and percentages indicated in the descriptions are purely indicative and may be subject to change or updates by the manufacturers. Due to the impossibility of updating these updates in real time, the photos and technical information of the products listed on farmaInnovation.it may differ from those shown on the label or otherwise disclosed by the manufacturers. The only identifying element is the MINSAN (Ministry of Health) code. Farmacia Barni, farmainnovation.it , does not guarantee the accuracy and timeliness of the information published and declines all responsibility for any errors, omissions, or failure to update it. farmainnovation.it assumes no responsibility for damages of any kind that may arise from accessing the published information.

